
Sodium hypochlorite has roots that reach back to the late 18th century, right in the middle of a wave of experimentation that changed how people handled sanitation and textiles. In the Paris suburb of Javel, chemists first churned out a weak solution that folks began calling “Eau de Javel.” Over time, sodium hypochlorite grew more prominent as its powerful bleaching and disinfecting properties proved useful both in industrial and domestic settings. During the cholera outbreaks of the 19th century, authorities started to spray the stuff in public spaces, hospitals, and waterworks. That long history of fighting disease and filth is a testament to the ways scientific curiosity sometimes spills over into essential community services.
Most people encounter sodium hypochlorite in the form of household bleach, though it pops up in plenty of commercial cleaning products, laundries, and even as a sanitizer for drinking water. Its distinctive smell and biting sting in the nose give it away right away. Strength differs depending on the application. Laundry bleach runs at concentrations around 5–6%, while swimming pool supplies and industrial goods can hit 12–15%. This flexibility gives manufacturers plenty of control, since there’s a version strong enough to clean mildew in a bathroom and another robust enough for food-processing plants. It’s hard to imagine any public or private cleaning operation without a regular shipment on hand.
Pour a glass of sodium hypochlorite solution and you’ll spot a pale yellowish tint. It tends to foam up if you shake it, and up close the acrid, chlorine-like odor makes it almost impossible to mistake for anything else. At room temperature, it flows smoothly like water, but don’t let that fool you. It’s an aggressive oxidizer and reacts in a flash when mixed with acids—often releasing hazardous chlorine gas. That same reactivity explains its purchasing restrictions and labeling requirements. As for shelf life, heat and light chip away at its potency, so storage always happens in opaque containers and cool storerooms. The chemical doesn’t accumulate in soil or water, which is a strength from an environmental viewpoint.
Any jug or drum of sodium hypochlorite that hits a retail shelf needs to announce concentrations clearly, partly to satisfy legal codes and partly to keep users safe. Manufacturers stamp every container with hazard symbols for corrosivity and indicate possible respiratory risks. The UN number for sodium hypochlorite is usually 1791 and you’ll spot GHS pictograms, precautionary statements, and recommended PPE instructions. Purity levels matter because even a splash of metal ions can trigger unwanted decomposition or reduce performance, so most suppliers promise tight batch control. Lot codes track every shipment from origin to shelf, a move that helps manufacturers trace back any problems during recalls or audits.
Most commercial batches of sodium hypochlorite start with the marriage of chlorine gas and a cold, dilute sodium hydroxide solution. This exothermic dance yields the hypochlorite solution along with a bit of salt and water. While chemists can tweak variables like concentration and temperature, the basic process rarely changes. The process must run inside a closed, ventilated system since chlorine leaks endanger workers and neighbors. Smaller labs sometimes rely on electrolysis of brine to whip up fresh stock on site, but those setups require careful monitoring to avoid running out of feedstock or throwing off concentrations.
Sodium hypochlorite acts as a generous oxidizer. Mix it with organic material or certain metals, and it triggers redox reactions that tear apart stains and pathogens. If it comes into contact with ammonia, the result is a toxic soup of chloramines—as anyone cleaning cat litter boxes while using bleach quickly learns. Acidic conditions flip hypochlorite into chlorine gas, the greenish-yellow vapor that strikes fear into anyone who’s ever studied industrial accidents. Alongside its common role in water treatment and bleaching, the compound can pick apart pharmaceuticals or organic pollutants through advanced oxidation processes, acting as the bouncer that breaks up stubborn contaminant “parties” at the molecular level.
Sodium hypochlorite can show up on labels as “liquid bleach,” “chlorine bleach,” “Javel water,” or in chemical catalogs under aliases like “NaOCl” and “hypochlorous acid, sodium salt.” Every hardware aisle in the world stacks up bottles labeled “bleach,” but strong industrial shipments often come marked with technical designations. Food-grade, disinfectant-grade, and textile-grade products may list slightly different concentrations or purity claims, which keeps purchasers on their toes. Those alternate names can cause confusion, so any facility using multiple sanitizers needs rigorous signage and training programs to avoid mixing products with dangerous results.
There’s no pretending sodium hypochlorite isn’t harsh. Direct skin contact burns; fumes irritate the lungs. Spill a bottle and any metal fittings soon start to rust and pit. OSHA and EPA handle much of the oversight, mandating gloves, goggles, and ventilation systems in workplaces that use or store large stocks. Any water treatment site or pool facility finds itself drawing up spill contingency plans and keeping neutralizing chemicals at hand. Industrial users track volumes carefully to sidestep environmental discharge limits. Most importantly, no one stores sodium hypochlorite near acids or ammonia to block those dangerous reactions that threaten more than just property damage.
Sodium hypochlorite’s reach runs from bathroom tiles to surgical wards. In municipal waterworks, operators depend on it for routine disinfection, giving millions easy access to safe drinking water. Hospitals and clinics trust it to wipe down surfaces that harbor pathogens like norovirus or C. difficile. Food processors turn to it for washing produce and sanitizing conveyor belts—always calibrating dose to kill contaminants but not taint food quality. Laundries rely on its bleaching muscle to scrub out tough stains in commercial linens. At poolside, sodium hypochlorite keeps water sparkling clear and free of algae blooms. During outbreaks of infectious diseases, public health crews step up dosing in communal spaces, and disaster response teams lean on it during floods and emergencies.
Chemists and engineers have given sodium hypochlorite plenty of attention over the years, mostly looking for ways to make it safer to handle, less likely to degrade, or more targeted in its pathogen kill rates. Research often focuses on improving formulas to resist the effects of storage without sacrificing disinfecting power. Newer stabilizers extend shelf life in hot climates. Scientists probe the molecules left behind on surfaces, making sure residue doesn’t interfere with food or medical devices. Environmental chemists look for alternatives that clean as well but break down even faster, hoping to dial back toxic byproducts. The challenge is always to balance disease-fighting might with safety for end users and nature.
Toxicologists keep track of sodium hypochlorite’s risks, which grow whenever misuse or accidental mixing brings out the worst in its chemistry. Acute exposure burns skin and eyes; inhaled vapors trouble the respiratory tract. Chronic exposure isn’t usually common given the compound’s reactive nature—most spills or splashes get neutralized, rinsed, or mopped up quickly. Still, research links long-term, high-level exposure with pulmonary irritation, asthma aggravation, or dental erosion in exposed cleaning staff. Environmental scientists monitor breakdown products like chlorate and trihalomethanes in waste streams, pushing for tight compliance with discharge limits to shield ecosystems. Education and clear labeling play a major part in preventing accidental poisonings from homemade cleaning blends.
Advances in green chemistry push researchers to either refine sodium hypochlorite or replace it where possible. The goal? Preserve its cheap, effective disinfection while reducing toxic aftermath and risks for workers and wildlife. Developments in sensor technologies help automate dosing in water plants and laundries, limiting waste and clumsy overuse. Formulators continue hunting for ways to reduce byproduct formation, improve storage stability, and ease safety burdens. In developing countries, on-site generation means more communities can secure safe water without shipping bulky drums of bleach. With each new outbreak or environmental regulation, scientists revisit sodium hypochlorite’s strengths and limits, making sure humanity’s reliance on sanitation doesn’t cause more harm than good in the long run.
Every time I reach for the bottle of bleach under my kitchen sink, I’m using sodium hypochlorite. This chemical, usually in a liquid form, works hard to disinfect, deodorize, and remove stains. It’s the most common ingredient in household bleach, cutting through grease and killing germs on countertops, bathrooms, and floors. During cold and flu season, people wipe down doorknobs and handles with it, trying to stop the spread of viruses and bacteria before anyone even thinks about getting sick.
Over the past few decades, sodium hypochlorite has become critical in keeping communities healthy in ways that are easy to overlook. Water treatment plants add carefully measured amounts to help purify drinking water. The Centers for Disease Control and Prevention credits this kind of disinfection with curbing diseases like cholera and typhoid. In swimming pools, lifeguards pour in sodium hypochlorite to prevent outbreaks of illnesses caused by parasites and bacteria. Without it, recreational water would quickly become unsafe.
Hospitals depend on sodium hypochlorite for more than mopping up floors. This chemical appears in protocols for cleaning surfaces that come into contact with contagious patients, lab benches after testing infectious samples, and even laundry destined for operating rooms. I’ve seen infection control officers stress about the right dilution—too strong, and it ruins fabric; too weak, and it can’t kill dangerous microorganisms like norovirus or Clostridioides difficile.
In food processing, sodium hypochlorite helps prevent foodborne illnesses. Factories use dilute solutions to wash fruit and vegetables and sanitize food preparation surfaces. No matter how fresh produce looks, dirt and invisible pathogens can cling to leaves and skins. By washing with a sodium hypochlorite solution, processors lower the risk of outbreaks and recalls. Grocery stores and restaurants also clean with it behind the scenes, protecting customers without most people realizing it.
Sodium hypochlorite’s power comes with its risks. Fumes can irritate eyes and lungs if a space isn’t ventilated. Mixed with acids or ammonia, it releases toxic gas. Manufacturers print warnings, but people sometimes forget to read those small labels. In my experience, school janitors and cafeteria workers need better training on chemical safety. Clear guidance and regular reminders help prevent accidents, especially for staff who clean after hours.
Using sodium hypochlorite brings up questions about what happens after its job is done. In water, it breaks down to salt and water, usually leaving little residue. But in large quantities or with improper disposal, it reacts with organic material to form harmful byproducts like trihalomethanes. Wastewater treatment plants monitor these chemicals, striving to keep them below regulatory limits. Investing in better monitoring and education about correct dilution and disposal goes a long way.
There are promising developments in safer, eco-friendly disinfectants. Hospitals test hydrogen peroxide vapor and ultraviolet light to reduce reliance on harsh chemicals. At home, simple soap and hot water clean many surfaces just as well for everyday use. Still, for emergencies and large-scale safety, sodium hypochlorite stands as a front-line defender. It serves as a reminder to keep exploring safer practices without losing sight of what keeps us healthy.
Many homes keep a bottle of bleach under the sink because it gets floors clean, wipes mold off shower tiles, and even brightens laundry. The magic behind most household bleach starts with sodium hypochlorite. This chemical kills bacteria, removes stains, and busts up viruses—it’s been part of cleaning routines for years. At the right concentration, sodium hypochlorite does a number on germs. That’s why schools, hospitals, and restaurants trust it for deep cleaning.
Still, ask five different people if sodium hypochlorite is safe, and you’ll get a mix of “definitely,” “never,” and “only with gloves.” Stories float around on social media about scary chemical burns or nasty fumes, which gets folks wondering how risky cleaning with bleach really is. Experience backs up some of these concerns. Last winter, after mopping my small bathroom with bleach and failing to open a window, I ended up feeling lightheaded and dizzy. A quick call to my local pharmacist confirmed that sodium hypochlorite in poorly-ventilated areas messes with the lungs.
At its core, sodium hypochlorite attacks the cell walls of microorganisms, so it kills a broad spectrum of bacteria and viruses. That’s exactly what hospitals love about it: super effective at stopping infection and disease spread. The Centers for Disease Control and Prevention, along with the Environmental Protection Agency, both confirm its value in controlling norovirus and other bad bugs around the kitchen or bathroom.
The catch? Straight out of the bottle, bleach contains a high concentration. For home use, mixing it with water brings the risk way down—usually about one-fourth cup per gallon of water for regular cleaning jobs. Direct use on surfaces can discolor grout and plastic, eat into fabric, and hurt your skin or eyes. I’ve had friends learn the hard way after getting splashes on their favorite shirts or forgetting the rubber gloves.
Too much sodium hypochlorite spells trouble. Bleach mixes especially poorly with anything acidic (think vinegar or some toilet cleaners) and releases toxic chlorine gas if combined. Breathing that stuff gets dangerous fast; even the CDC puts out clear warnings against mixing cleaning products. Weekly news stories about accidental fumes speak to how these mistakes still happen in real-world kitchens.
On top of that, constant contact with full-strength bleach dries out your hands, causes burns, and triggers asthma attacks in sensitive people. The American Lung Association warns that fumes irritate the lungs, and even mild exposure creates headaches for allergy sufferers. Pets, too, can have problems after accidental contact—I've seen a neighbor call the vet after her dog walked across a freshly mopped tile floor.
Sodium hypochlorite doesn’t need to be banished from the home. Paid attention to the label, always opened a few windows, and wore gloves when scrubbing bathroom tiles. Keeping the bottle away from acids, sealing it up tight between uses, and keeping it out of kids’ reach covers the basics. Mixing fresh solution each time avoids chemical breakdown, since sunlight or heat can zap its cleaning power. The stuff works wonders on countertops and floors if handled with respect. Simple habits can prevent most problems—the payoff is a cleaner, safer home without extra worry.
Sodium hypochlorite helps keep water clean and fights germs in many settings, including homes, pools, and hospitals. Every bottle or tank comes with a label warning about the risks, but these warnings often go ignored. I’ve seen janitorial closets where open bleach bottles sit next to acids—an accident just waiting to happen. It’s more than just a smelly mess if spills or fumes escape; breathing problems or skin burns can follow. Safety isn’t just about following rules—nobody wants to be the one explaining a chemical mishap.
Storing sodium hypochlorite really means staying ahead of decay and leaks. In warm areas, this chemical can break down faster and lose its strength. Cool, well-ventilated spaces slow the chemical’s breakdown. Sunlight plays the villain here; rays slice right through the solution’s power. You want a dark spot for storage, away from any heat sources or sparks.
I’ve learned through years of maintenance work that storing bleach in metal containers guarantees rust and gas. Over time, sodium hypochlorite chews up metal, especially steel and aluminum. Plastic tanks made from high-density polyethylene or fiberglass keep the solution safe and stable much longer. Even so, chemical-resistant liners in storage rooms help prevent any long-term corrosion or damage.
Mix sodium hypochlorite with ammonia or acids, and you have a recipe for a toxic gas leak. I once saw a pool attendant store all the cleaning chemicals on one shelf—one bump and chlorine fumes filled the air. Keep this chemical in its own locked area, away from acids, oils, and flammable liquids. Posting clear warning signs on the storage room door helps remind staff and rescue workers what’s inside.
Good air flow keeps fumes from building up. A fan or vented window can save you from headaches—sometimes quite literally. Stable shelving and secondary containment trays add another shield against leaks or drips. Even if a jug falls over, the spill has somewhere to go don’t end up on the floor or worse, mixing with an incompatible substance.
Even with the best habits, things can go wrong. In my experience, spill kits placed right outside the storage area make cleanup quick and safe. Every kit should have personal protective gear—gloves, goggles, aprons—along with proper absorbents like sand or soda ash. Once, a cracked bottle seeped into cardboard and started eating through. Quick action, with the right gear, stopped a minor mess from turning into a major incident.
The people handling sodium hypochlorite often rotate or multitask. Relying on one training session a year doesn’t cut it. Refresher lessons make sure workers know not only storage protocols but also emergency procedures if something spills or fumes start to build up. Real-world practice in using protective gear or spill kits means fewer surprises if trouble hits.
Sodium hypochlorite, most people recognize it as household bleach. You see it on grocery store shelves, and you probably have a bottle under your sink. The power of this liquid comes with risks and responsibilities. Using it straight from the bottle burns skin and damages surfaces. Diluting it right protects people, homes, and public spaces.
Health organizations around the world recommend clear concentrations for sodium hypochlorite. The CDC, for instance, tells us to mix common household bleach (that usually has 5%–6% sodium hypochlorite) at a 1:10 ratio for disinfecting surfaces with blood or body fluids. That means adding one part bleach to nine parts water. For standard hard surface disinfection, especially during outbreaks of viruses and bacteria, a 1:49 or 1:50 dilution (that’s 20 milliliters bleach in one liter of water) works for most cleaning needs in homes, schools, and clinics.
A lot of people assume “stronger is better.” That’s not the case. Undiluted or over-concentrated mixtures put children, pets, and adults at risk for chemical burns and breathing troubles. I still remember my neighbor getting sick because she used too much bleach while cleaning during early COVID days. The smell drove her out of her own apartment, and she had to call her son for help. If you mix it right, you get germ-killing power with less risk.
Tables, door handles, kitchen counters, and even laundry hold up better against diluted bleach. Splashing strong bleach onto a kitchen counter can strip shine and harm grout, while proper dilution keeps cleaning effective. After years of talking to cleaning professionals, I learned many went through gloves and ruined uniforms quickly because they ignored guidance and worked with harsh solutions. Proper dilution saves money and reduces environmental harm, since less bleach washes down the drain.
Measuring bleach isn’t rocket science. A simple kitchen measuring cup or even an old water bottle works. If you have to clean up something like vomit or blood, a stronger mix (1:10) is necessary. For day-to-day surface cleaning against bacteria and viruses, a 1:50 dilution does the job. Make solutions fresh every day. Bleach and water mixes lose strength over time—especially if left in sunlight—so don’t rely on old mixtures.
Hospitals and large facilities often print out charts to help staff mix things right. Households can do the same, even taping instructions next to the cleaning cupboard. Schools should hold short chats with cleaners so everyone knows the safe way. I’ve found that people are more likely to get it right if the instructions are simple, visual, and shared as a group routine, not just a rule in a booklet.
Disinfecting isn’t about using more chemicals. It’s about using what works, at the right strength, with practical habits. Stick to trusted ratios, measure carefully, and talk about bleach safety the same way you’d talk about allergic reactions, fire safety, or safe driving. Only then can we protect people and the surfaces we trust every day.
Sodium hypochlorite pops up in homes, hospitals, water treatment plants, and more. It shines as a disinfectant, killing off bacteria and viruses that stubbornly hang around on surfaces. That clear, almost innocuous-looking liquid packs real punch as a cleaner—yet that same strength can cause serious harm if treated carelessly.
I spent years working in a lab setting where sodium hypochlorite solutions were a daily tool. Even seasoned professionals show respect when handling it. A single spill can eat through clothes, burn the skin, and wreck equipment. Everyone remembers the first whiff up close—a sharp, nose-stinging reminder of what happens when you don’t pay attention to proper handling methods.
Nobody argues with goggles or face shields after witnessing a splash burn. Eyes don’t stand a chance against sodium hypochlorite. Gloves—nitrile or neoprene, not bare hands—form the first line of defense. Lab coats or aprons prevent lingering drops from seeping into clothing. These aren't just suggestions—they’re the only reason some folks avoid permanent scars or eye damage.
Many folks meet sodium hypochlorite’s fumes for the first time during a botched cleanup at home or in a janitor’s closet. The chemical gives off chlorine gas, especially when mixed with acids or ammonia. With poor airflow, headaches or respiratory problems kick in fast. In closed areas, simple tasks become real dangers. Keeping windows open and running fans aren’t just tick-box exercises—they save lungs and prevent fainting spells.
I’ve learned you can never assume what came through a container before sodium hypochlorite gets poured in. Even rinse water with a trace of acid will react with sodium hypochlorite, releasing toxic chlorine gas before you realize it. Storing the chemical away from direct sunlight, heat, or incompatible substances like ammonia means one less reason for an emergency cleanup. Mixing with other cleaners is a gamble that countless accident reports warn against.
Pouring slowly, double-checking labels, and never trusting your memory about what’s safe—these steps turn habit into self-preservation.
Many workplaces cut corners with training. In my experience, walking someone through safety sheets beats every warning sign posted. Emergency eyewash stations and showers gather dust until the day someone needs them. Drills make a difference. Knowing what to do when there’s a spill, how to use neutralizers, and understanding why you call a supervisor before trying to fix a problem on your own creates a safety buffer you can’t buy in a bottle.
Tossing leftover sodium hypochlorite in the nearest drain can wreck plumbing and hurt the environment. Communities post clear rules for disposal, often including neutralization steps or special collection days. Keeping containers sealed and upright, far from food storage or heat sources, stops leaks before they spoil an entire storeroom.
Responsibility doesn’t end after closing the bottle. Clear labeling, safe storage, and thoughtful disposal keep homes, workplaces, and water supplies free from accidental pollution and injury. Safe use of sodium hypochlorite starts with awareness—and that’s something everyone can practice, whether in a big facility or a kitchen cupboard.
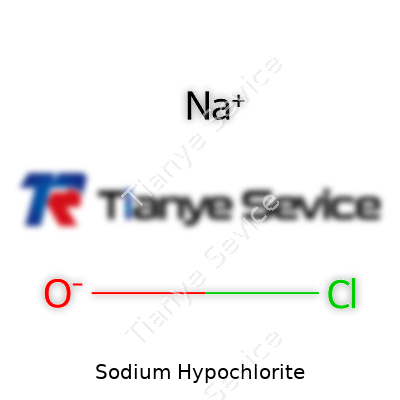

| Names | |
| Preferred IUPAC name | Sodium hypochlorite |
| Other names |
Liquid bleach
Bleach Javel water Antiformin Soda bleach |
| Pronunciation | /ˌsoʊdiəm haɪpəˈklɔːraɪt/ |
| Identifiers | |
| CAS Number | 7681-52-9 |
| Beilstein Reference | 3587269 |
| ChEBI | CHEBI:32146 |
| ChEMBL | CHEMBL1201080 |
| ChemSpider | 14110 |
| DrugBank | DB09130 |
| ECHA InfoCard | 100.028.765 |
| EC Number | 231-668-3 |
| Gmelin Reference | Gmelin Reference: 14408 |
| KEGG | C02338 |
| MeSH | D017959 |
| PubChem CID | 23665760 |
| RTECS number | NH3486307 |
| UNII | 9G4X8W3A0K |
| UN number | 1791 |
| Properties | |
| Chemical formula | NaOCl |
| Molar mass | 74.44 g/mol |
| Appearance | Clear, pale yellow to greenish liquid |
| Odor | Chlorine-like |
| Density | 1.11 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.72 |
| Vapor pressure | <1 mmHg (20°C) |
| Acidity (pKa) | 7.5 |
| Basicity (pKb) | pKb ≈ 14 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.33 |
| Viscosity | 10-12 cP |
| Dipole moment | 2.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 107.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -345.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -123 kJ/mol |
| Pharmacology | |
| ATC code | D08AX09 |
| Hazards | |
| Main hazards | Causes severe skin burns and eye damage; may cause respiratory irritation; harmful if swallowed. |
| GHS labelling | GHS02, GHS05, GHS09, GHS07 |
| Pictograms | GHS05,GHS09 |
| Signal word | DANGER |
| Hazard statements | H290: May be corrosive to metals. H314: Causes severe skin burns and eye damage. H400: Very toxic to aquatic life. |
| Precautionary statements | P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P330, P337+P313, P391, P501 |
| NFPA 704 (fire diamond) | Health: 3, Flammability: 0, Instability: 1, Special: - (oxidizer OX sometimes noted) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Rat oral 8,910 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 8,917 mg/kg |
| NIOSH | NIOSH: **NH9450000** |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Hypochlorite: "2 mg/m³ (as chlorine, OSHA PEL) |
| REL (Recommended) | 0.5% solution |
| IDLH (Immediate danger) | IDLH: 10 mg/m³ |
| Related compounds | |
| Related compounds |
Sodium chlorate
Sodium chloride Calcium hypochlorite Potassium hypochlorite Lithium hypochlorite |